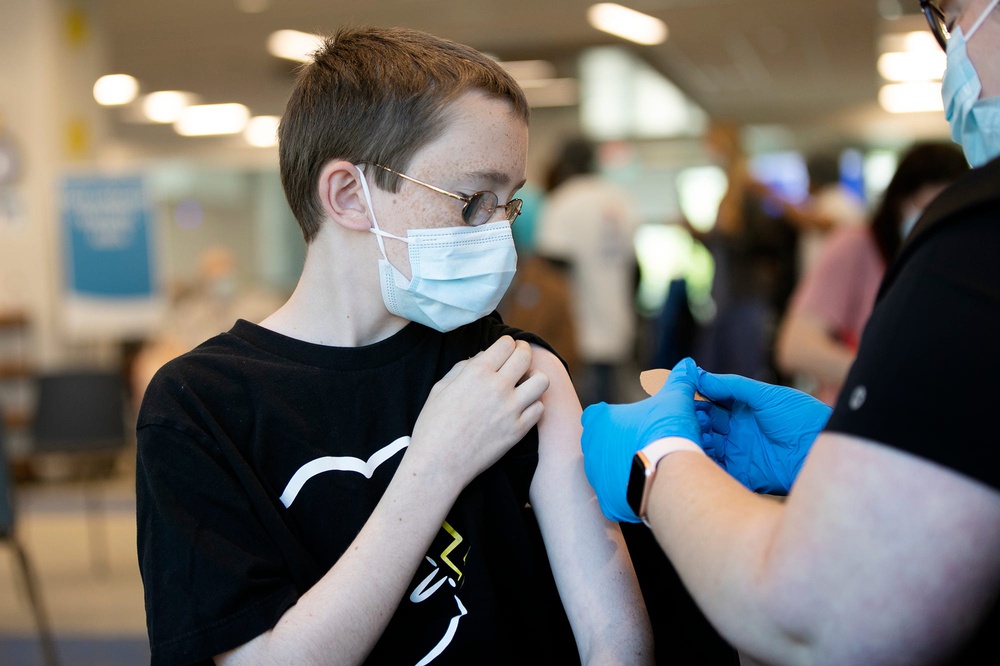
Matthew Schmersal, 12, gets his first COVID-19 shot, administered by RN Amy Vereb, at OhioHealth Blom Administrative Campus, Thursday May 13, 2021. OhioHealth started vaccinations of kids ages 12 to 15, following the FDA's approval of the Pfizer COVID-19 vaccine for anyone ages 12 and up. 02 Teen Vaccines Clh
Syndication: The Columbus Dispatch
SILVER SPRING, MD (77WABC) – An FDA panel has authorized the Pfizer vaccine for elementary school-aged children ages 5 to 11.
The decision comes after the agency reviewed data released by Pfizer that showed a kid-sized, two-dose regimen of the COVID vaccine is safe in children in that age bracket.
The FDA must still grant final approval of the vaccine.
Pending approval , a CDC panel will consider the safety of Pfizer’s vaccine for elementary school children beginning next week,.
If approved, the vaccine could be available by early to mid November.














